What We Offer
Prenatal Diagnosis via Amniocentesis and Chorionic Villus Sampling (CVS)
For everyone, regardless of age, the minimum risk of a chromosomal or copy number variant problem is at least 1%. In many situations, it is often higher. CVS or amniocentesis is performed to obtain fetal tissues for analysis. These procedures have identified chromosomal abnormalities, such as Down syndrome, and neural tube defects, such as spina bifida. Recently, however, explosive growth in DNA testing technology has made it possible to detect literally hundreds of additional genetic disorders, including Mendelian disorders such as Tay-Sachs, cystic fibrosis, Duchenne muscular dystrophy, and sickle-cell anemia, to name just a few for which there is a change in the DNA sequence of those genes to alter them. We also now can look for small gains and losses of chromosomal material called “copy number variants” that produce conditions such as Cri-du-Chat, Angelman, Prader Willi, Williams, DiGeorge, and hundreds of others that collectively are more common than Down syndrome.
We employ molecular technologies that allow us to obtain results for certain conditions such as Down Syndrome, Trisomies 13 and 18, and the sex chromosomes 1-2 days after the procedure.
Amniocentesis was the first method developed for prenatal diagnosis – dating back to the late 1960s and early 1970s. It is usually performed between 15 and 20 weeks from the beginning of the last menstrual period. Under ultrasound guidance a thin needle is inserted through the woman's abdominal wall and into the uterus and amniotic cavity. The procedure feels like having blood drawn. Approximately one ounce of fluid is withdrawn and sent to the laboratory for analysis. In experienced hands, genetic amniocentesis is a very safe test. A significant limitation of amniocentesis, however, is that reassurance or diagnosis of problems does not occur until the mid to late second trimester.

Chorionic Villus Sampling (CVS)
Chorionic villus sampling, or CVS, is the first-trimester procedure for prenatal diagnosis of fetal chromosomal and genetic disorders, usually performed 11 to 13 weeks from the beginning of the last menstrual period although in some instances it can be used much later in pregnancy. The procedure is done one of two ways. In singleton pregnancies, most of the time (about 70% of the time for us) we place the patient in stirrups, and a speculum is inserted just as for a Pap smear. A small plastic catheter (similar to a straw) is passed painlessly through the cervix and maneuvered under ultrasound guidance into the placental tissue. A syringe is then attached to the end of the catheter, and a very small amount of placental tissue is aspirated. In some cases (about 30% of the time for singletons), depending upon the position of the placenta, a needle may be inserted transabdominally, like an amniocentesis, and maneuvered into the placenta (not the fluid). Most American women given the choice between a “Pap smear” like experience or a needle, prefer the “Pap Smear.” In experienced operators, however, do as many as 90% of cases abdominally, because they do not have the experience to perform the vaginal approach.
In either case, several milligrams of tissue are obtained and sent to the lab. Tests can then be performed for chromosomal (e.g. Down syndrome) molecular diagnosis (such as cystic fibrosis, or FISH for a quick read of chromosomes such as Down syndrome), or molecular analysis (for Mendelian and copy number variant disorders).
We commonly perform CVS for diagnosis of singleton pregnancies:
1. For everyone (regardless of maternal age), as the background risk of a problem is at least 1% for everyone, and for others at even higher risk for birth defects; for diagnosis
2. in multiple pregnancies prior to reduction, to help ensure the health of fetuses remaining after the procedure; and
3. for patients who have undergone preimplantation genetic testing (PGT) with in vitro fertilization because they need post-implantation confirmation of the PGT results.
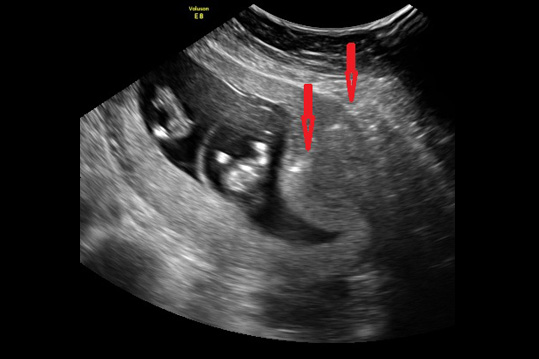
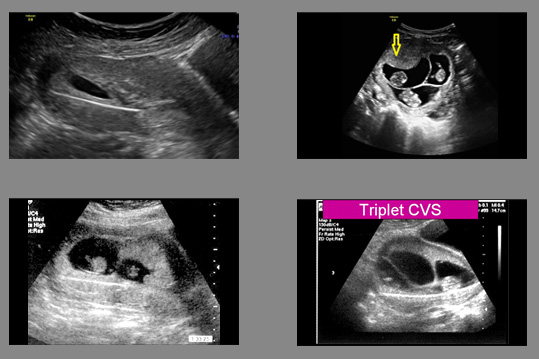
Safety of Procedures
For many years, it was a common misconception that amniocentesis was a safer procedure than CVS. Multiple studies have now confirmed (what those of us who have been doing CVS have known for decades) - that CVS is just as safe or safer than amniocentesis. Some operators have quoted amniocenteses risks at 1/1500 or 1/100 depending upon whether they were for or against. Both such extremes are not realistic. On the basis of having done more than 60,000 procedures, in very experienced hands we quote risks of both procedures at about 1/1000 over and above the background risk of spontaneous loss. CVS is especially useful in multiple pregnancies because if a reduction is being considered it is much safer to be done at 12 weeks rather than waiting until about 20 weeks. Furthermore, with recent attacks on the availability of the right to an abortion including lowering the gestational age at which it can be performed, it is best to get answers as early as possible.
Fetal Tissue Sampling
Tests utilizing the DNA in fetal tissues have made possible via CVS the diagnosis of disorders such as Duchenne muscular dystrophy. Sometimes, however, DNA tests cannot give a definitive answer, and the only way to get the answer is by obtaining a piece of fetal muscle, or liver for analysis of its tissue structure or protein analysis within the tissue. Over the past 30 years, we have performed more fetal muscle biopsies than anyone else, worldwide.
Similarly, some skin abnormalities and certain chromosomal abnormalities can only be diagnosed by obtaining a piece of fetal skin. Fetal skin biopsy is the optimal way to evaluate ambiguity that occasionally arises in the interpretation of amniocentesis data. These biopsies are performed without a hospital stay, and under ultrasound guidance.
Laboratory Testing
There has been a virtual explosion in the sophistication of laboratory testing available both for screening tests as described above and for direct evaluation of fetal health from CVS, amniocentesis, fetal skin, and fetal tissues.
For the past 70 years the traditional test for chromosomal abnormalities has been the karyotype. Cells from tissue specimens are cultured and then the chromosomes are organized into groups based upon their size and the centromere which connects a "long" and a "short" arm. There are normally 46 of these – actually 23 pairs. Each of us normally inherits one of each of our parent's chromosomes which pairs up with one from the other parent. When we make our sperm or eggs, one of each of our pairs goes into the sperm or egg.
Chromosomes contain our genes, which are the instructions (blue print) necessary for proper organ formation, growth, and development. The usual number of chromosomes for humans is 46, or 23 pairs. Normally, each person inherits half of their chromosomes from their mother and half from their father. The first 22 pairs of chromosomes are numbered (1 to 22) and the last pair is given a letter (X or Y). Women usually get an X from mom and from dad – XX. Men get an X from mom and a Y from dad – XY. Each chromosome from mom matches up with one from dad so in the photo below, one of the number 1s is from the mother and the other is from the father. One of the number 2s is from the mother and the other number 2 is from the father, and so on. The numbered chromosomes are the same for men and women. The lettered chromosomes determine sex. If there is no Y, the person is typically female. If there is a Y, the person is typically male.
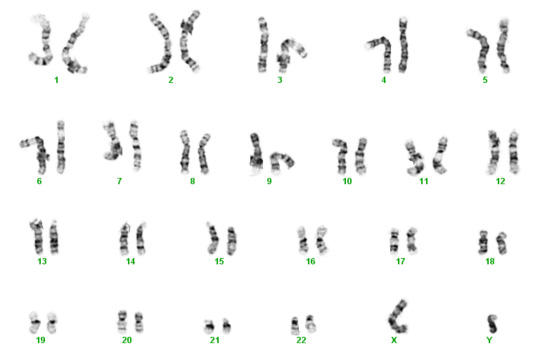
Unfortunately, biological mistakes sometimes happen. The most common scenario is that in the process of creating eggs for fertilization, the splitting of one of the mother's pair doesn't occur, leading to an egg with two copies instead of one. Then with fertilization, the embryo/fetus has three instead of two.
Below are two karyotypes. The first is of a male with Trisomy 21, more commonly known as Down syndrome. The second karyotype below is a female with Trisomy 18 – a condition with a very high mortality rate and severe disabilities in surviving infants.
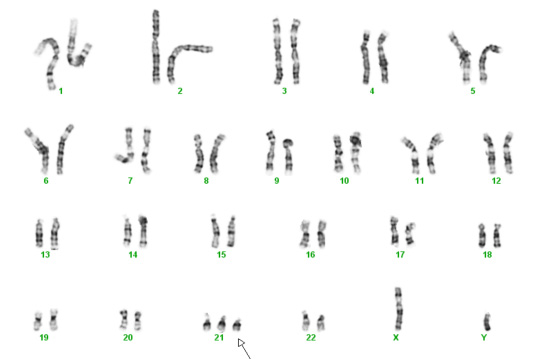
Trisomy 21
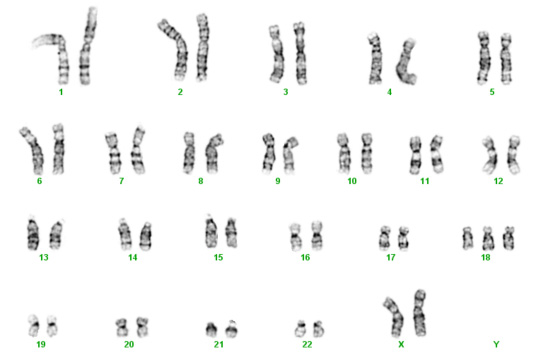
Trisomy 18
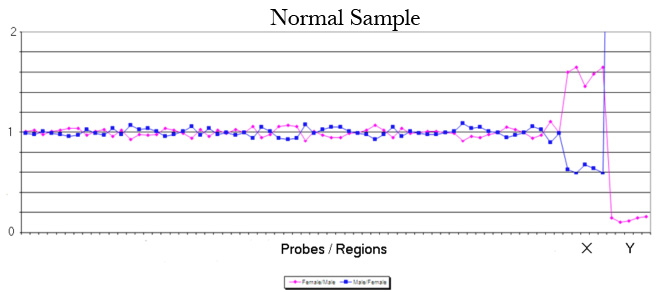
Because our patients are "average New Yorkers" who want their answers yesterday, in addition to the karyotype we also run molecular testing with a technique called fluorescent in situ hybridization (FISH) that gives rapid answers for chromosome 21 (Down syndrome), trisomies 13 and 18, and the sex chromosomes looking for abnormalities but which gives gender as a byproduct. The two figures below show a normal male. The first picture has two copies of Chromosomes 18, one "X" and one "Y." The second shows two copies of chromosomes 13 and 21. The bottom figure shows 3 copies of chromosome 21 and represents Down syndrome.

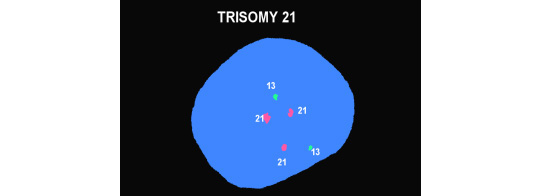
Microarrays
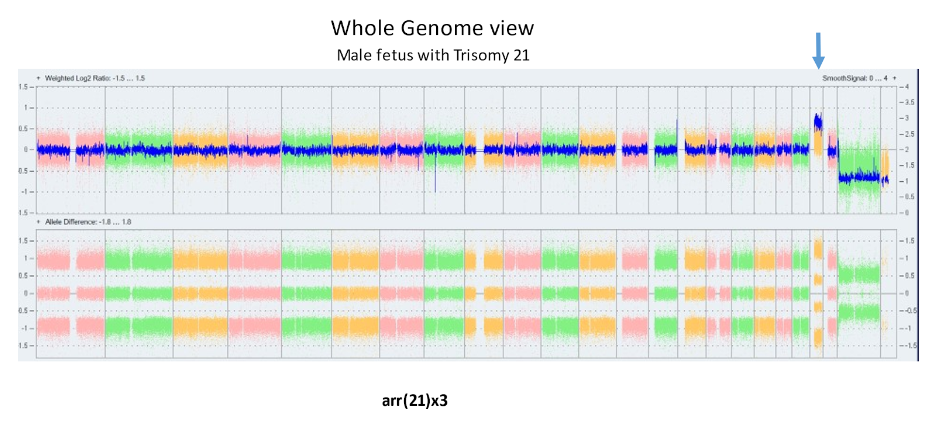
The laboratory technique of array CGH (aCGH), often called microarray, offers more precise identification of additions or deletions of material. aCGH can identify changes far too small to be seen on karyotype. The smallest piece of a chromosome that can generally be seen on karyotype has about 5 – 7 million base pairs. The aCGH typically gets to a resolution of near 200 thousand. Thus, it can be thought of as a 30 fold magnification over the karyotype. It can also get down to much higher resolutions when appropriate. For pediatricians, microarray replaced the karyotype a decade ago. Microarrays will replace prenatal karyotypes as the primary diagnostic test for chromosomes. Below are array depictions of a normal male – one X chromosome and one Y chromosome. The second using a more modern display shows three copies of chromosome 21 in a male fetus.
In the 2000’s, the general consensus was to restrict aCGH use to those cases in which there was an ultrasound abnormality and a NORMAL karyotype. The 2012 NICHD study showed nearly 6% of such cases have an abnormality of significance on the microarray. The same study demonstrated that for patients with a normal ultrasound having CVS or amniocentesis because of advanced maternal age or because of increased risk on screening tests, the additional detection of significant gain or loss of material is about 1/100 (1.0%) when there is a normal karyotype. The risk is not a function of maternal age. Since this risk number is, in fact, higher than the risk quoted to a 35 year old for traditional aneuploidy (1/200, 0.5), we began in 2012 years ago offering aCGH to ALL PATIENTS REGARDLESS OF THEIR ACTUAL AGE.
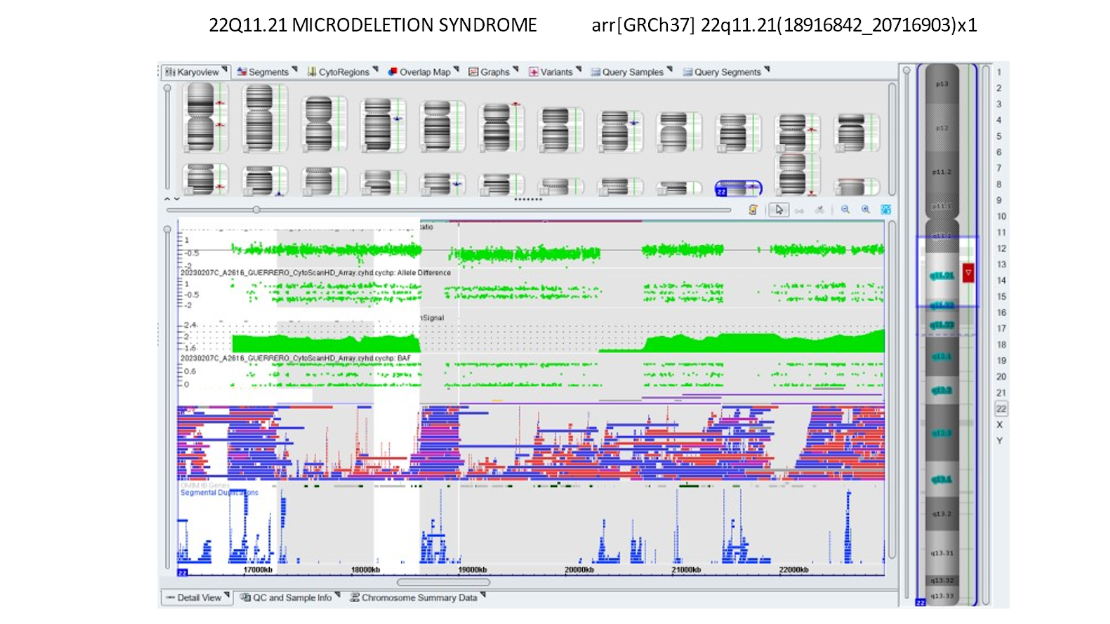
Our own data and publications have shown about a 1% incidence of findings of significance in patients who had no other reason to be considered at high risk. Below is a microarray showing DiGeorge syndrome which is a deletion on 22q.
Whole Exome Sequencing is the “next step” in higher magnification for great detail. It sequences the 1-2% of the genome that is actively functioning. Beyond that will be Whole Genome Sequencing which looks at the remaining 98% that is typically not active but can cause occasional problems. The same progression of using the “new test” when there is an ultrasound anomaly but the existing laboratory tests (FISH, Karyotype, and now microarray don’t demonstrate what it is) will lead to the new one being used. Experience to date shows the “new test” can find an anomaly between 5-20% of cases. Eventually, that test becomes “routine,” the next one becomes the leading-edge research one.
With every emerging technology there is a period in which certain findings on tests cannot be clearly determined to be abnormal or not. We have seen this, for example, for multiple ultrasound abnormalities such as the choroid plexus cyst and the echogenic focus. During this time, we have "numerators" before we have the "denominators." We now know that some patients have an addition or deletion of material which we know or strongly believe is benign. However, the chance of having a variation of uncertain significance – meaning we don’t know if it is of clinical significance or not is also about 0.5%. This number continues to fall with increasing experience. For now, patients have to decide whether the increased pick up is worth the potential for increased anxiety without an answer in small percentages of patients. Insurance coverage for microarrays often unquestioned for many companies but occasionally can be problematic. The same pattern is seen for most genetic tests.
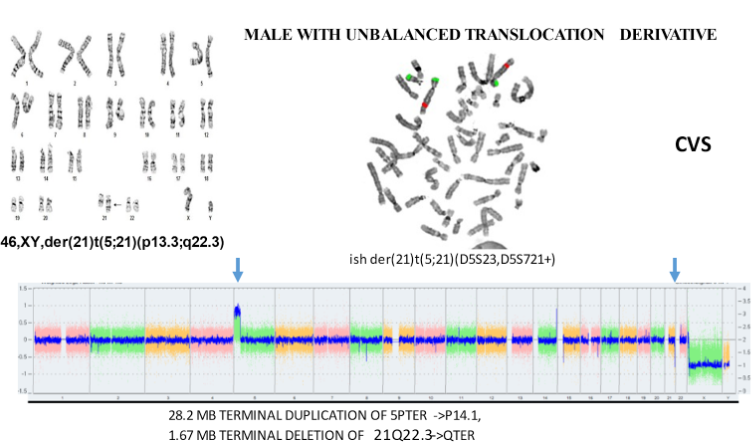
The above aCGH shows an unbalanced translocation between chromosomes 13 and 18 that would be expected to have serious consequences for the fetus.
We offer these arrays in almost all situations.
Fetal Therapy
The diagnosis of a serious disorder in a fetus is a devastating event for all involved. In many cases, there may be little that medicine can offer, aside from help in preparing for the birth of child with special needs, or the option to terminate the pregnancy. In some situations, however, there is another alternative: treating the disorder before birth.
Over the past 4 decades, Dr. Evans has been one of the major developers of fetal therapy. His contributions include:
- developed the first method for preventing a congenital defect before birth (congenital adrenal hyperplasia);
- performed the first successful stem cell transplant to cure a baby with SCIDS (the "bubble babies");
- was a member of the team that did the first successful open fetal surgery (for congenital diaphragmatic hernia); and
- is one of the most experienced at performing fetal shunt procedures, such as for obstructed fetal bladders. We work with colleagues all over the country to provide cutting-edge therapies in pregnancies with correctable problems.
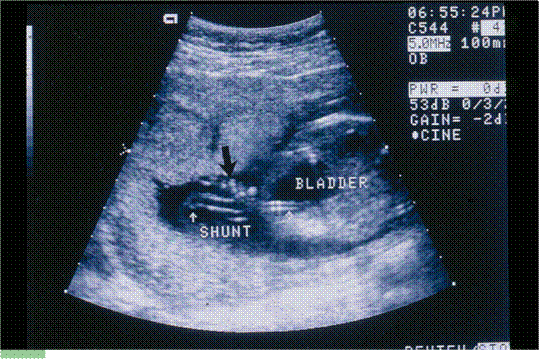
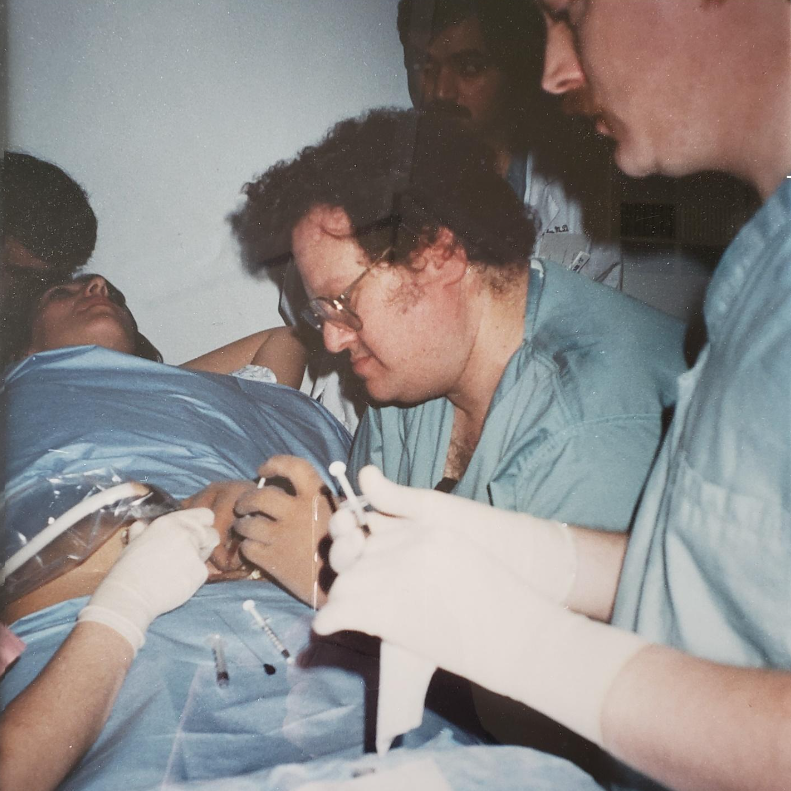
Dr. Evans performing the first successful in utero stem cell transplant at 14 weeks’ gestation to cure a baby with X-Severe combined immune deficiency (bubble baby – no immunity) in 1995.
Subnavigation
Evans Pioneer Award Lecture
-
Quick Contact Info
Dr. Mark I. Evans (MD PLLC)
Phone: 212.288.1422
Fax: 212.879.2606
Email: Evans@CompreGen.com
131 E 65TH ST
NEW YORK NY 10065

